As a professional writing services company for the Life Sciences and Pharmaceutical industry, TRA360 attempts to stay current with recent scientific developments and shares them in our blog.
More than a century ago, Dr. Paul Erlich discovered certain chemicals are selectively absorbed by tissues and they could be a delivery vehicle for drugs to the disease site. [ref]Wang, Jeffrey, Shen, Wei-Chiang and Zaro, (Eds.),- Antibody-Drug Conjugates; The 21st Century Magic Bullets for Cancer, Springer 2015[/ref] In cancer therapy, monoclonal antibodies (mAbs) have shown fewer side effects compared to chemotherapy. And a combination of mAbs and chemicals often brings even more efficacy and fewer side effects. Antibody Drug Conjugates (ADCs) are created by attaching mAbs to biologically active drugs with chemical linkers.
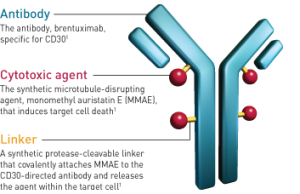
Each ADC has three important parts: a) antibody b) chemical molecule c) linker. Despite this simple concept, discovering each specific part and, most importantly, conjugating them, is a complex but promising field in life sciences. Early ADCs were too toxic or not enough effective or linkers were not stable in the blood.
The industry’s belief in ADCs is high and there are more than 100 ADCs in the development pipelines of pharmaceutical companies. Currently, only two drugs; ADCETRIS® for treatment of Hodgkin’s lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL) and KADCYLA® for treatment of HER2-positive metastatic breast cancer (MBC) are available on the market. Seattle Genetics and ImmunoGen are on the edge of development of these drugs and many ADCs researchers are using this scientific methodology. In 2014, Roche had 10 molecules in clinical development.[ref]http://www.reportlinker.com/p02280919/Antibody-Drug-Conjugates-Market-Edition.html[/ref]
There are also current co-development agreements in the market. A consortium of Chinese companies including Shanghai Fosun Pharmaceutical Group, HOPU Investment Management, China Everbright Limited’s healthcare fund (“CEL Healthcare Fund”), and WuXi PharmaTech have started negotiations to acquire Ambrx. ADCs are the main reason for this targeted acquisition. Ambryx is anticipating clinical trial evaluation of ARX-788, an ADC that targets HER2 over-expressing tumors including breast, gastric, colon, pancreatic and ovarian cancers in 2015.[ref]http://ambrx.com/chinese-consortium-enters-into-agreement-to-acquire-ambrx[/ref]
Another example is Mersana Therapeutics, a Cambridge MA-based biotech company that specializes in immunoconjugate therapies with 6 ADCs in its pipeline. The company has development agreements with Takeda and EMD Serono for two of them.
The development of commercially viable ADCs is still in its early stages and the FDA is expecting to approve two more by 2020. As new products continue to move through the phases of clinical trials and if the results are successful in treating people with life-threatening and terminal diseases, the future looks promising for patients and investors.
Are you a health professional with an interest in ADCs? Do you have any comments on this article? Let us know here or tweet at us @TRA360!
